Are We All Pre-Diabetic?
 Even if a doctor assures you that your blood sugar is "normal," alarming evidence documents that you are at significant risk of premature death unless you achieve optimal 24-hour-a-day glucose control.
Even if a doctor assures you that your blood sugar is "normal," alarming evidence documents that you are at significant risk of premature death unless you achieve optimal 24-hour-a-day glucose control.
Life Extension® long ago warned of the silent dangers when fasting blood sugar exceeds 85 mg/dL. New scientific studies validate this position.
Even more insidious are data showing that blood sugar "spikes" that occur after each meal dramatically increase the risk of cardiovascular disease, retinal damage, and cancer.
Unless steps are taken to suppress after-meal sugar surges, every large meal you eat can trigger a dangerous metabolic cascade that results in cell damage and accelerated aging.
Fortunately, proven methods exist to support optimal blood sugar throughout the day.
The latest is a green coffee bean extract that targets a critical enzyme involved in after-meal blood sugar spikes. When tested on humans in a placebo-controlled study, this natural extract produced an astounding 32% drop in after-meal blood sugar!1
An Epidemic of Elevated Blood Sugar
 The percentage of adults who suffer chronic high blood sugar is staggering!
The percentage of adults who suffer chronic high blood sugar is staggering!
One report evaluated 46,000 middle-age individuals and found more than 80% had fasting blood sugar of 85 mg/dL or greater.2
Another study involving 11,000 middle-age and older individuals showed more than 85% had fasting blood sugar of 85 mg/dL or greater.3
Since incidence of disease starts to increase when fasting blood sugar rises above these levels, this means the vast majority of aging humans today endure chronic cellular damage associated with elevated blood sugar.
This epidemic of elevated blood sugar will accelerate age-related disease until the medical profession realizes that their test values for defining "normal" blood sugar are horrifically defective.
Mainstream Medicine's Fatal Flaws
 There are two major problems with the way mainstream medicine views blood sugar.
There are two major problems with the way mainstream medicine views blood sugar.
First, the "normal" range for fasting blood sugar is still set too high. Current criteria specify that you are not "diabetic" unless fasting blood glucose exceeds 125 mg/dL. The range between 100 and 125 mg/dL is considered "pre-diabetic."4 In other words, with a blood sugar reading of 99 mg/dL, your doctor will tell you everything is fine and send you home ignorant of the dangers lurking within your body.
Recall that optimal glucose control occurs when fasting blood glucose is kept between 70 and 85 mg/dL. This means that fasting glucose levels that doctors accept today as "normal" are in reality dangerously elevated.
Second, mainstream doctors do not tell their patients about the risks of after-meal (postprandial) blood sugar spikes. After every meal, these sudden surges in blood sugar damage delicate blood vessels in your brain, heart, kidneys, and eyes, as well as accelerate the aging of cells and tissues throughout your body.
Utilizing only fasting blood glucose readings does not detect perilous after-meal glucose spikes that present an increased risk of death.5,6 The critical truth is that standard definitions of diabetes are dangerously outdated.
 Scientific research shows that after-meal spikes in blood sugar are potentially more damaging than elevations of fasting blood sugar.7-10 For example, in people with "normal" blood sugars and "normal" glucose tolerance tests, the risk of a heart attack increases by 58% for each 21 mg/dL increase in after-meal blood sugar.11 And for a similar after-meal glucose elevation, risk of dying from cardiovascular disease increases by 26%.12 Control of after-meal glucose needs to be recognized as a critical component in reduction of cardiovascular complications.13
Scientific research shows that after-meal spikes in blood sugar are potentially more damaging than elevations of fasting blood sugar.7-10 For example, in people with "normal" blood sugars and "normal" glucose tolerance tests, the risk of a heart attack increases by 58% for each 21 mg/dL increase in after-meal blood sugar.11 And for a similar after-meal glucose elevation, risk of dying from cardiovascular disease increases by 26%.12 Control of after-meal glucose needs to be recognized as a critical component in reduction of cardiovascular complications.13
What doctors don't realize is that an isolated fasting glucose reading fails to provide information on their patients' glucose control throughout the day. So when a patient has a fasting glucose reading of, let's say, 95 mg/dL, this may be an artificially low number that does not reflect the patient's real-world, all-day glucose status that may be considerably higher.
For instance, a patient with a 95 mg/dL fasting glucose reading may spend most of their day significantly above 150 mg/dL, as their aging body is unable to neutralize the impact of the excess calories they chronically ingest.
Without controlling fasting and postprandial sugar spikes, the stage is set for accelerated aging and a series of degenerative diseases.
Why We Are Predisposed to Elevated Glucose
 If blood sugar ever drops too low, death rapidly ensues as brain cells cannot function long without adequate glucose. To protect against acute death in the near-starvation state our ancestors encountered, the body developed compensatory mechanisms to ensure that glucose levels don't fall too low.
If blood sugar ever drops too low, death rapidly ensues as brain cells cannot function long without adequate glucose. To protect against acute death in the near-starvation state our ancestors encountered, the body developed compensatory mechanisms to ensure that glucose levels don't fall too low.
The problem with these protective mechanisms is that they cause the majority of aging people today to suffer dangerously elevated blood sugar, as widespread famine no longer exists in modern societies.
How Chronic Glucose Overload Occurs
 While glycogenolysis protected our ancestors from acute starvation and death, the impact on people today is the opposite. As we age, this internal control mechanism (glycogenolysis) malfunctions, resulting in dangerously high blood glucose levels.
While glycogenolysis protected our ancestors from acute starvation and death, the impact on people today is the opposite. As we age, this internal control mechanism (glycogenolysis) malfunctions, resulting in dangerously high blood glucose levels.
Another factor causing elevated glucose involves excess creation of new glucose in the body. In healthy people, a biochemical process known as gluconeogenesis creates new glucose from other substances such as amino acids when blood sugar levels are too low. Aging people often make too much glucose from all kinds of foods, even when blood glucose levels are already too high.
Contrary to popular belief, carbohydrates are not the only food source of blood sugar. Amino acids found in protein readily convert to blood glucose via gluconeogenesis.
An enzyme involved in gluconeogenesis and glycogenolysis is glucose-6-phosphatase.
With increasing age and rising blood sugar levels, control of glucose-6-phosphatase can become impaired. When this occurs, glucose-6-phosphatase increases the release of stored glucose from the liver (glycogenolysis) and enhances glucose creation (gluconeogenesis)…despite already sufficient after-meal blood sugar levels.
Excess activity of glucose-6-phosphatase is why many aging people find it nearly impossible to achieve optimal glucose levels. Compounds that target glucose-6-phosphatase are being aggressively sought to counter a diabetes epidemic that has nearly tripled in the United States over the past three decades.
 Since conventional medical authorities are still not diagnosing patients as "diabetic" until they show fasting glucose over 125 mg/dL or after-meal glucose over 199 mg/dL, the true number of people today whose health is being destroyed by high blood sugar remains grossly underestimated.
Since conventional medical authorities are still not diagnosing patients as "diabetic" until they show fasting glucose over 125 mg/dL or after-meal glucose over 199 mg/dL, the true number of people today whose health is being destroyed by high blood sugar remains grossly underestimated.
For example, data indicate the risk for stroke increases as fasting glucose increases above 83 mg/dL. In fact, every 18 mg/dL increase beyond 83 results in a 27% greater risk of dying from stroke.14 So failing to aggressively lower glucose levels is exposing the vast majority of the American population to the leading cause of long-term disability and third highest cause of death, i.e., stroke.
Even adherence to a calorie-reduced diet, or a low-carbohydrate diet, does not always protect against skyrocketing glucose levels triggered through glucose-6-phosphatase.
To summarize, the two internal factors that conspire to elevate glucose levels are:
- Glycogenolysis—Release of stored glucose from the liver.
- Gluconeogenesis—Creation of new glucose from non-carbohydrates.
Excess expression of glucose-6-phosphatase is involved in glycogenolysis and gluconeogenesis. Suppression of glucose-6-phosphatase offers a crucial strategy for limiting the destructive impact of elevated after-meal blood sugar.
Combating Excess Glucose with Chlorogenic Acid
 In the quest for natural ways to safely inhibit after-meal blood sugar spikes, scientists turned their attention to plant compounds that target the glucose-6-phosphatase enzyme.
In the quest for natural ways to safely inhibit after-meal blood sugar spikes, scientists turned their attention to plant compounds that target the glucose-6-phosphatase enzyme.
Pharmaceutical researchers have been eager to discover a drug that would target after-meal blood sugar spikes.15,16 Ideally, this drug would suppress glucose-6-phosphatase, which is involved in new glucose formation and release of glucose from its storage site in the liver.15-18
Exciting new findings show a natural compound within the green coffee bean known as chlorogenic acid can modulate after-meal blood sugar surges.
As a multitude of studies show, coffee consumption is associated with a reduced risk of type 2 diabetes.19-23 We now understand chlorogenic acid in coffee exerts significant anti-diabetic properties. And as recent studies show, 80-85% of the adult population is at risk for diabetic complications because their blood glucose levels are too high!2
Green Coffee Extract Improves Glucose Control
 Green coffee bean extract found in unroasted coffee beans, once purified and standardized, produces high levels of chlorogenic acid and other beneficial polyphenols that can suppress excess blood glucose levels.
Green coffee bean extract found in unroasted coffee beans, once purified and standardized, produces high levels of chlorogenic acid and other beneficial polyphenols that can suppress excess blood glucose levels.
Human clinical trials support the role of chlorogenic acid-rich green coffee bean extract in promoting healthy blood sugar control and reducing disease risk.
Aware of the crucial importance of controlling after-meal blood sugar spikes, scientists conducted a study among 56 healthy volunteers, challenging them with an oral glucose tolerance test before and after a supplemental dose of green coffee extract. The oral glucose tolerance test is a standardized way of measuring a person's after-meal blood sugar response.
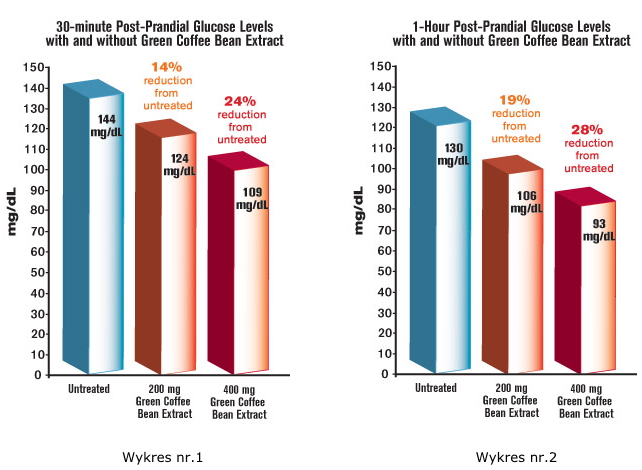 In subjects not taking green coffee bean extract, the oral glucose tolerance test showed the expected rise of blood sugar to an average of 144 mg/dL after a 30 minute period. But in subjects who had taken 200 mg of the green coffee bean extract, that sugar spike was significantly reduced, to just 124 mg/dL—a 14% decrease1 (See figure 1).
In subjects not taking green coffee bean extract, the oral glucose tolerance test showed the expected rise of blood sugar to an average of 144 mg/dL after a 30 minute period. But in subjects who had taken 200 mg of the green coffee bean extract, that sugar spike was significantly reduced, to just 124 mg/dL—a 14% decrease1 (See figure 1).
That impressive difference was sustained throughout the two-hour study period at a dose as low as 200 mg of green coffee bean extract. Subjects had a mean 19% reduction of bloodsugar at one hour, and a 22% reduction (glucose down to just 89 mg/dL) at two hours, compared to each patient's own untreated levels. In other words, the amount of time subjects had glucose levels in the dangerous range was sharply curtailed by the green coffee bean extract1 (See figures 1 and 2).
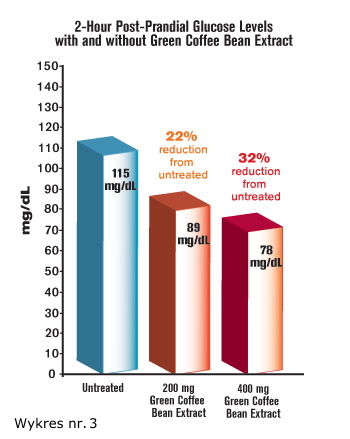 To state this differently, when subjects did not take the green coffee bean extract, their oral glucose tolerance reading after two hours showed blood sugar of 115 mg/dL—a higher-than-desirable level. In response to a modest dose of 200 mg of green coffee bean extract, two-hour blood sugar levels dropped to only 89 mg/dL1 (See figure 3).
To state this differently, when subjects did not take the green coffee bean extract, their oral glucose tolerance reading after two hours showed blood sugar of 115 mg/dL—a higher-than-desirable level. In response to a modest dose of 200 mg of green coffee bean extract, two-hour blood sugar levels dropped to only 89 mg/dL1 (See figure 3).
For most aging individuals, even after eating nothing for eight to twelve hours, it is challenging to achieve a "fasting" glucose reading as low as 89 mg/dL. Yet when these study subjects took 200 mg of green coffee bean extract, their glucose levels dropped to 89 mg/dL just two hours after drinking a pure glucose solution. The high-dose glucose drink used in standard oral glucose tolerance tests spikes blood sugar more than typical meals.
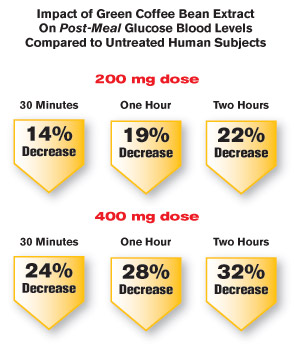
When a higher dose (400 mg) of green coffee bean extract was supplemented before an oral glucose challenge test there was an even greater average reduction in blood sugar—up to nearly 28% at one hour!1
How Green Coffee Bean Extract Suppresses Glucose Elevation
Scientists have discovered that chlorogenic acid found abundantly in green coffee bean extract inhibits the enzyme glucose-6-phosphatase that triggers new glucose formation and glucose release by the liver.25,26 As discussed earlier, glucose-6-phosphatase is involved in dangerous after-meal spikes in blood sugar.27
Another means by which chlorogenic acid acts to suppress after-meal glucose surges is by inhibiting alpha-glucosidase. This intestinal enzyme breaks apart complex sugars and enhances their absorption into the blood.28 Slowing the absorption of common sugars (including sucrose) limits after-meal glucose spikes.29
In yet another significant mechanism, chlorogenic acid increases the signal protein for insulin receptors in liver cells.30 That has the effect of increasing insulin sensitivity, which in turn drives down blood sugar levels.
 Chlorogenic acid-rich plant extracts have been shown to reduce fasting blood glucose values by more than 15% in diabetic patients with poor response to medication.31 A similar effect was seen in healthy volunteers, whose intestinal absorption of glucose was reduced following a chlorogenic acid-enriched coffee drink.32
Chlorogenic acid-rich plant extracts have been shown to reduce fasting blood glucose values by more than 15% in diabetic patients with poor response to medication.31 A similar effect was seen in healthy volunteers, whose intestinal absorption of glucose was reduced following a chlorogenic acid-enriched coffee drink.32
When a chlorogenic acid supplement of 1 gram was given before meals, glucose levels were reduced by 13 mg/dL, just 15 minutes after an oral glucose challenge, demonstrating its ability to rapidly lower the after-meal blood sugar spike in humans.33
In a clinical trial, researchers gave different dosages of green coffee bean extract, standardized for chlorogenic acid, to 56 people. Thirty-five minutes later, they gave the participants 100 grams of glucose in an oral glucose challenge test. Blood sugar levels dropped by an increasingly greater amount as the test dosage of green coffee bean extract was raised, from 200 mg up to 400 mg. At the 400 mg dosage, there was a full 24% decrease in blood sugar—just 30 minutes after glucose ingestion.1
This means that if you had a dangerous after-meal glucose reading of 160 mg/dL, green coffee bean extract would slash it to 121 mg/dL.
These findings are in line with supportive data demonstrating green coffee bean extract's numerous blood sugar-reducing mechanisms of action.
Other experimental models reveal that chlorogenic acid favorably modulates gene expression to enhance the activity of liver cells and increase levels of the hormone adiponectin, which enhances insulin sensitivity and exerts anti-inflammatory, anti-diabetic, and anti-atherogenic effects.34
Benefits and Limitations of High Coffee Consumption
 Many epidemiologic studies suggest a benefit of consuming large amounts of coffee. Moderate to high amounts of coffee consumption is associated with a sharply reduced risk of developing type 2 diabetes.58-61 Laboratory studies suggest that coffee may have anti-tumor effects against ovarian, colon, liver, and other cancers.62-66 And coffee consumption may be associated with a decreased risk of stroke in women, while those who consume moderate amounts of coffee may be protected against acute coronary syndromes.67,68
Many epidemiologic studies suggest a benefit of consuming large amounts of coffee. Moderate to high amounts of coffee consumption is associated with a sharply reduced risk of developing type 2 diabetes.58-61 Laboratory studies suggest that coffee may have anti-tumor effects against ovarian, colon, liver, and other cancers.62-66 And coffee consumption may be associated with a decreased risk of stroke in women, while those who consume moderate amounts of coffee may be protected against acute coronary syndromes.67,68
For many people, drinking large amounts of coffee is not ideal.
Large amounts of caffeine may be irritating. One study found that people who drank 12 or more cups of coffee daily were getting a total of 960-1,380 mg of caffeine.69,70 For many individuals, high levels of caffeine consumption can be undesirable.
Additionally, it is important to note that the commercial process of roasting coffee beans creates a molecule called HHQ, that actually reduces some of the activity of the chlorogenic acid found in green coffee.71,72
A very low-caffeine extract derived from unroasted green coffee bean provides a standardized dose of beneficial chlorogenic acid.
Disease Risks of High-Normal Blood Sugar
 Cancer: Numerous studies—including one published in the May 17, 2010, online issue of The Oncologist that was so large that it included half of all type 2 diabetics in Sweden35—found that, separate from the known risks for cancer among diagnosed diabetics,36-38 the risk for some cancers escalated directly with blood sugar levels, even among those without diabetes. Rising in lock-step with glucose levels as they edged up within the normal range were the risks for cancers of the endometrium,39 pancreas,40 colon,41,42 and colorectal tumors of a more aggressive nature.43
Cancer: Numerous studies—including one published in the May 17, 2010, online issue of The Oncologist that was so large that it included half of all type 2 diabetics in Sweden35—found that, separate from the known risks for cancer among diagnosed diabetics,36-38 the risk for some cancers escalated directly with blood sugar levels, even among those without diabetes. Rising in lock-step with glucose levels as they edged up within the normal range were the risks for cancers of the endometrium,39 pancreas,40 colon,41,42 and colorectal tumors of a more aggressive nature.43
Cardiovascular disease: Subjects showed risks for cardiovascular events, cardiovascular disease, and cardiovascular mortality that increased in direct relation to elevated—but still high-normal—glucose levels.44-48 One researcher commented that within limits, the lower the glucose levels—even among those without diabetes—the lower the cardiovascular risk. Coronary artery disease risk was twice as high in patients with post-meal glucose levels between 157 and 189  mg/dL compared to those with levels under 144 mg/dL.49 While diabetes is defined as experiencing regular after-meal glucose levels of 200 mg/dL, one research team found a risk for stroke that increased as fasting glucose levels rose above 83 mg/dL. In fact, every 18 mg/dL increase beyond 83 resulted in a 27% greater risk of dying from stroke.14
mg/dL compared to those with levels under 144 mg/dL.49 While diabetes is defined as experiencing regular after-meal glucose levels of 200 mg/dL, one research team found a risk for stroke that increased as fasting glucose levels rose above 83 mg/dL. In fact, every 18 mg/dL increase beyond 83 resulted in a 27% greater risk of dying from stroke.14
Cognitive impairment: As blood sugar rose—whether within the normal or the diabetic ranges—the risk for this mild cognitive impairment and dementia increased.50,51
Kidney disease: Surges in blood sugar promoted a greater production of fibrous kidney tissue—which causes kidney disease—than a high but constant blood sugar level.52 The study authors suggested it may be fluctuations in glucose—more than the levels themselves—that produce the vascular complications implicated in kidney damage. Another study found a direct increase in chronic kidney disease as levels of hemoglobin A1c (a marker of long-term glucose control) rose.53
Pancreatic dysfunction: Beta cells located in the pancreas produce the insulin that helps control blood sugar. But high glucose levels can make these cells dysfunctional, raising the risk of type 2 diabetes. Researchers discovered that mild beta cell dysfunction was already detectable in those whose glucose levels spiked two hours after eating, despite staying completely within the range considered by the medical establishment to be normal.54
 Diabetic retinopathy: High glucose levels precipitate diabetic retinopathy—damage to the retina that can lead to blindness. In one study, retinopathy was diagnosed in 13% of people who later progressed to diabetes and in 8% of those who never progressed to diabetes.55
Diabetic retinopathy: High glucose levels precipitate diabetic retinopathy—damage to the retina that can lead to blindness. In one study, retinopathy was diagnosed in 13% of people who later progressed to diabetes and in 8% of those who never progressed to diabetes.55
Neuropathy: As expected, patients with nervous system damage (neuropathy) whose postprandial (after-meal) glucose readings were above the diabetic threshold, showed damage to their large nerve fibers. However, neuropathy patients whose glucose readings—although elevated—remained well within the normal range still showed damage to their small nerve fibers. Within any blood sugar range, reported the journal Neurology in 2003, the higher the glucose, the greater the involvement of the large nerve fibers.56 Another nerve damage study in 2006 confirmed these results.57
 Green coffee bean extract contains a natural compound (chlorogenic acid) that reduces blood sugar by inhibiting the glucose-6-phosphatase enzyme.
Green coffee bean extract contains a natural compound (chlorogenic acid) that reduces blood sugar by inhibiting the glucose-6-phosphatase enzyme.
Glucose-6-phosphatase increases blood sugar by promoting the creation of new glucose (gluconeogenesis) and inducing the release of glucose stored in the liver (glycogenolysis).
Those with fasting glucose above 85 mg/dL, or whose oral glucose tolerance test shows two-hour postprandial glucose surges over 125 mg/dL, should consider taking 200 to 400 mg of standardized green coffee bean extract two to three times daily, thirty-five minutes before meals.
To clarify the postprandial (after-meal) risk, if your fasting glucose is 85 mg/dL, but an oral glucose tolerance test shows blood glucose above 125 mg/dL after two hours, then you are likely to be suffering from toxic after-meal glucose surges that can be neutralized by taking green coffee bean extract before most meals.
Most aging people suffer both fasting and postprandial glucose overload, and should initiate steps to suppress the dangerous glucose-6-phosphatase enzyme.
Summary
The need to redefine diabetes is critical because risk of premature death and disease rises sharply with fasting blood sugar greater than 85 mg/dL.
Furthermore, the insidious impact of after-meal blood sugar spikes goes largely undetected, exposing the vast majority of people to accelerated disease risk.
Behind this danger is the little-appreciated role of glucose-6-phosphatase in creating and releasing additional glucose into the blood. This enzyme, which helps regulate blood sugar when you're young, can inappropriately trigger a dangerous after-meal surge of blood sugar as you age.
Avant-garde scientists have identified a breakthrough weapon to control these surges: green coffee bean extract. This natural ingredient contains a compound called chlorogenic acid shown to target glucose-6-phosphatase and blunt post-consumption blood sugar levels by up to 32%.
A consistent finding in those who restrict their calorie intake is markedly lower blood glucose levels. Longevity enthusiasts can now benefit from a novel yet natural green coffee bean extract to combat internal processes that cause dangerous elevations in blood glucose.
Material used with permission of Life Extension. All rights reserved.
1. Nagendran MV. Effect of green coffee bean extract (GCE), High in Chlorogenic Acids, on Glucose Metabolism. Poster presentation number: 45-LB-P. Obesity 2011, the 29th Annual Scientific Meeting of the Obesity Society. Orlando, Florida. October 1-5, 2011.
2. Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008 Jun;121(6):519-24.
3. Kato M, Noda M, Suga H, Matsumoto M, Kanazawa Y. Fasting plasma glucose and incidence of diabetes—implication for the threshold for impaired fasting glucose: results from the population-based Omiya MA cohort study. J Atheroscler Thromb. 2009;16(6):857-61.
4. Available at: http://diabetes.niddk.nih.gov/dm/pubs/diagnosis/#diagnosis. Accessed August 15, 2011.
5. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999 Aug 21;354(9179):617-21.
6. Nakagami T. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia. 2004 Mar;47(3):385-94.
7. Miura K, Kitahara Y, Yamagishi S. Combination therapy with nateglinide and vildagliptin improves postprandial metabolic derangements in Zucker fatty rats. Horm Metab Res. 2010 Sep;42(10):731-5.
8. Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care. 2008 Feb;31 Suppl 2:S150-4.
9. Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol. 2008 Nov;2(6):1094-100.
10. Triggle CR. The early effects of elevated glucose on endothelial function as a target in the treatment of type 2 diabetes. Timely Top Med Cardiovasc Dis. 2008;12:E3
11. Gerstein HC, Pais P, Pogue J, Yusuf S. Relationship of glucose and insulin levels to the risk of myocardial infarction: a case-control study. J Am Coll Cardiol. 1999 Mar;33(3):612-9.
12. Lin HJ, Lee BC, Ho YL, et al. Postprandial glucose improves the risk prediction of cardiovascular death beyond the metabolic syndrome in the nondiabetic population. Diabetes Care. 2009 Sep;32(9):1721-6.
13. Yu PC, Bosnyak Z, Ceriello A. The importance of glycated haemoglobin (HbA(1c)) and postprandial glucose (PPG) control on cardiovascular outcomes in patients with type 2 diabetes. Diabetes Res Clin Pract. 2010 Jul;89(1):1-9.
14. Batty GD, Kivimäki M, Smith GD, Marmot MG, Shipley MJ. Post-challenge blood glucose concentration and stroke mortality rates in non-diabetic men in London: 38-year follow-up of the original Whitehall prospective cohort study. Diabetologia. 2008 July;51(7):1123-6.
15. Hemmerle H, Burger HJ, Below P, et al. Chlorogenic acid and synthetic chlorogenic acid derivatives: novel inhibitors of hepatic glucose-6-phosphate translocase. J Med Chem. 1997 Jan 17;40(2):137-45.
16. Arion WJ, Canfield WK, Ramos FC, et al. Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose 6-phosphatase. Arch Biochem Biophys. 1997 Mar 15;339(2):315-22.
17. Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010 Nov;59(11):2697-707.
18. Henry-Vitrac C, Ibarra A, Roller M, Merillon JM, Vitrac X. Contribution of chlorogenic acids to the inhibition of human hepatic glucose-6-phosphatase activity in vitro by Svetol, a standardized decaffeinated green coffee extract. J Agric Food Chem. 2010 Apr 14;58(7):4141-4.
19. Salazar-Martinez E, Willett WC, Ascherio A, et al. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med. 2004 Jan 6;140(1):1-8.
20. Pereira MA, Parker ED, Folsom AR. Coffee consumption and risk of type 2 diabetes mellitus: an 11-year prospective study of 28,812 postmenopausal women. Arch Intern Med. 2006 Jun 26;166(12):1311-6.
21. Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003 Oct;78(4):728-33.
22. Bidel S, Hu G, Sundvall J, Kaprio J, Tuomilehto J. Effects of coffee consumption on glucose tolerance, serum glucose and insulin levels–a cross-sectional analysis. Horm Metab Res. 2006 Jan;38(1):38-43.
23. van Dam RM, Feskens EJM. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2002 Nov 9;360(9344):1477-8.
24. Murase T, Misawa K, Minegishi Y, Aoki M, Ominami H, Suzuki Y, Shibuya Y, Hase T. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am J Physiol Endocrinol Metab. 2011 Jan;300(1):E122-33.
25. Henry-Vitrac C, Ibarra A, Roller M, Merillon JM, Vitrac X. Contribution of chlorogenic acids to the inhibition of human hepatic glucose-6-phosphatase activity in vitro by Svetol, a standardized decaffeinated green coffee extract. J Agric Food Chem. 2010 Apr 14;58(7):4141-4.
26. Andrade-Cetto A, Vazquez RC. Gluconeogenesis inhibition and phytochemical composition of two Cecropia species. J Ethnopharmacol. 2010 Jul 6;130(1):93-7.
27. Bassoli BK, Cassolla P, Borba-Murad GR, et al. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: effects on hepatic glucose release and glycaemia. Cell Biochem Funct. 2008 Apr;26(3):320-8.
28. Ishikawa A, Yamashita H, Hiemori M, et al. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J Nutr Sci Vitaminol (Tokyo). 2007 Apr;53(2):166-73.
29. Alonso-Castro AJ, Miranda-Torres AC, Gonzalez-Chavez MM, Salazar-Olivo LA. Cecropia obtusifolia Bertol and its active compound, chlorogenic acid, stimulate 2-NBDglucose uptake in both insulin-sensitive and insulin-resistant 3T3 adipocytes. J Ethnopharmacol. 2008 Dec 8;120(3):458-64.
30. Rodriguez de Sotillo DV, Hadley M, Sotillo JE. Insulin receptor exon 11+/- is expressed in Zucker (fa/fa) rats, and chlorogenic acid modifies their plasma insulin and liver protein and DNA. J Nutr Biochem. 2006 Jan;17(1):63-71.
31. Herrera-Arellano A, Aguilar-Santamaria L, Garcia-Hernandez B, Nicasio-Torres P, Tortoriello J. Clinical trial of Cecropia obtusifolia and Marrubium vulgare leaf extracts on blood glucose and serum lipids in type 2 diabetics. Phytomedicine. 2004 Nov;11(7-8):561-6.
32. Thom E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J Int Med Res. 2007 Nov-Dec;35(6):900-8.
33. van Dijk AE, Olthof MR, Meeuse JC, Seebus E, Heine RJ, van Dam RM. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care. 2009 Jun;32(6):1023-5.
34. Zhang LT, Chang CQ, Liu Y, Chen ZM. Effect of chlorogenic acid on disordered glucose and lipid metabolism in db/db mice and its mechanism. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2011 Jun;33(3):281-6.
35. Hemminki K, Li X, Sundquist J, Sundquist K. Risk of cancer following hospitalization for type 2 diabetes. The Oncologist. 2010;15(6):548-55. Epub 2010 May 17.
36. Czyzyk A, Szczepanik Z. Diabetes mellitus and cancer. Eur J Intern Med. 2000 Oct;11(5):245-52.
37. Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009 Dec;16(4):1103-23.
38. Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Metformin and cancer: doses, mechanisms and the dandelion and hormetic phenomena. Cell Cycle. 2010 Mar 21;9(6):1057-64.
39. Cust AE, Kaaks R, Friedenreich C, Bonnet F, et al. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition EPIC. Endocr Relat Cancer. 2007 Sep;14(3):755-67.
40. Rosato V, Tavani A, Bosetti C, et al. Metabolic syndrome and pancreatic cancer risk: a case-control study in Italy and meta-analysis. Metabolism. 2011 May 5.
41. Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999 Jul 7;91(13):1147-54.
42. Aleksandrova K, Boeing H, Jenab M, et al. Metabolic syndrome and risks of colon and rectal cancer: the European Prospective Investigation into Cancer and Nutrition Study. Cancer Prev Res (Phila). 2011 Jun 22.
43. Healy L, Howard J, Ryan A, et al. Metabolic syndrome and leptin are associated with adverse pathological features in male colorectal cancer patients. Colorectal Dis. 2011 Jan 20.
44. Pan WH, Cedres LB, Liu K, et al. Relationships of clinical diabetes and symptomatic hyperglycaemia to risk of coronary heart disease mortality in men and women. Am J Epidemiol. 1986;123:504-16.
45. Wilson PWF, Cupples LA, Kannel WB. Is hyperglycaemia associated with cardiovascular disease? The Framingham Study. Am Heart J. 1991 Feb;121(2 Pt 1):586-90.
46. de Vegt F, Dekker JM, Ruhe HG, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn study. Diabetologia. 1999 Aug;42(8):926-31.
47. Saydah SH, Miret M, Sung J, Varas C, Gause D, Brancati FL. Post-challenge hyperglycemia and mortality in a national sample of U.S. adults. Diabetes Care. 2001;24:1397-402.
48. Coutinho M, Gerstein H, Poque J, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events: a meta regression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–40.
49. Donahue RP, Abbott RD, Reed DM, et al: Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987 Jun;36 (6):689-92.
50. Tali Cukierman-Yaffe T, Gerstein HC, Williamson JD. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009 Feb;32(2):221-6.
51. Sonnen JA, Larson EB, Brickell K. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009 Mar;66(3):315-322.
52. Polhill TS, Saad S, Poronnik S, Fulcher GR, Pollock CR. Short-term peaks in glucose promote renal fibrogenesis independently of total glucose exposure. Am J Physiol Renal Physiol. 2004 Aug;287(2):F268-73.
53. Bash, LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident kidney disease even in the absence of albuminuria and retinopathy: atherosclerosis risk in communities (ARIC) study. Arch Intern Med. 2008 Dec 8/22;168(22):2440-7.
54. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, De Fronzo RA, San Antonio metabolism study. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004 Jan;47(1):31-9.
55. Available at: http://docnews.diabetesjournals.org/content/2/8/1.2.full. Accessed August 16, 2011.
56. Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003 Jan 14;60(1):108-11.
57. Hoffman-Snyder C; Smith BE; Ross MA; Hernandez J; Bosch EP. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol. 2006 Aug;63(8):1075-9.
58. van Dam RM, Feskens EJ. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2002 Nov 9;360(9344):1477-8.
59. Rosengren A, Dotevall A, Wilhelmsen L, Thelle D, Johansson S. Coffee and incidence of diabetes in Swedish women: a prospective 18-year follow-up study. J Intern Med. 2004 Jan;255(1):89-95.
60. Huxley R, Lee CM, Barzi F, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009 Dec 14;169(22):2053-63.
61. Salazar-Martinez E, Willett WC, Ascherio A, et al. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med. 2004 Jan 6;140(1):1-8.
62. Butt MS, Sultan MT. Coffee and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 2011 Apr;51(4):363-73.
63. Granado-Serrano AB, Martin MA, Izquierdo-Pulido M, Goya L, Bravo L, Ramos S. Molecular mechanisms of (-)-epicatechin and chlorogenic acid on the regulation of the apoptotic and survival/proliferation pathways in a human hepatoma cell line. J Agric Food Chem. 2007 Mar 7;55(5):2020-7.
64. Kang NJ, Lee KW, Kim BH, et al. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis. 2011 Jun;32(6):921-8.
65. Rakshit S, Mandal L, Pal BC, et al. Involvement of ROS in chlorogenic acid-induced apoptosis of Bcr-Abl+ CML cells. Biochem Pharmacol. 2010 Dec 1;80(11):1662-75.
66. Tai J, Cheung S, Chan E, Hasman D. Antiproliferation effect of commercially brewed coffees on human ovarian cancer cells in vitro. Nutr Cancer. 2010;62(8):1044-57.
67. Larsson SC, Virtamo J, Wolk A. Coffee consumption and risk of stroke in women. Stroke. 2011 Apr;42(4):908-12.
68. Panagiotakos DB, Pitsavos C, Chrysohoou C, Kokkinos P, Toutouzas P, Stefanadis C. The J-shaped effect of coffee consumption on the risk of developing acute coronary syndromes: the CARDIO2000 case-control study. J Nutr. 2003 Oct;133(10):3228-32.
69. Zhang Y, Lee ET, Cowan LD, Fabsitz RR, Howard BV. Coffee consumption and the incidence of type 2 diabetes in men and women with normal glucose tolerance: the Strong Heart Study. Nutr Metab Cardiovasc Dis. 2011 Jun;21(6):418-23.
70. Available at: http://www.ico.org/caffeine.asp. Accessed July 21, 2011.
71. Suzuki A, Fujii A, Yamamoto N, et al. Improvement of hypertension and vascular dysfunction by hydroxyhydroquinone-free coffee in a genetic model of hypertension. FEBS Lett. 2006 Apr 17;580(9):2317-22.
72. Yamaguchi T, Chikama A, Mori K, et al. Hydroxyhydroquinone-free coffee: a double-blind, randomized controlled dose-response study of blood pressure. Nutr Metab Cardiovasc Dis. 2008 Jul;18(6):408-14.


