Curcumin. Novel Turmeric Compound Delivers Much More Curcumin to the Blood part 1
 Life Extension readers have long been aware of the vast array of health benefits conferred by the curry spice turmeric, which is the source of curcumin. Scientific researchers around the world are investigating applications for curcumin that include fighting cancer, arthritis, diabetes, cardiovascular disease, osteoporosis, and reversing the pathological processes underlying Alzheimer’s disease, among other conditions.1-13
Life Extension readers have long been aware of the vast array of health benefits conferred by the curry spice turmeric, which is the source of curcumin. Scientific researchers around the world are investigating applications for curcumin that include fighting cancer, arthritis, diabetes, cardiovascular disease, osteoporosis, and reversing the pathological processes underlying Alzheimer’s disease, among other conditions.1-13
Curcumin has long been known to have poor bioavailability, requiring high doses to achieve desired blood levels. A novel curcumin absorption system has been developed that delivers up to seven times more pharmacologically bioactive curcumin to the blood compared with commercial curcumin products.
This revolutionary development will radically change the extent to which people may obtain additional health benefits from this revered herb.
A wealth of data shows that curcumin and its related chemicals (collectively known as curcuminoids) help to prevent and fight a wide range of diseases—from cancer to cardiovascular disease—through a variety of mechanisms.1,5,7-19 These include powerful anti-inflammatory, antioxidant, chemopreventive (cancer-preventive), and antineoplastic (cancer-fighting) properties.
Perhaps one of curcumin’s most important activities in the human body is its ability to inhibit activation of the transcription factor, nuclear factor-kappa B (NF-kB),4-6 a potent inducer of chronic inflammation. NF-kB is a protein that acts as a sort of switch, turning on inflammation by activating genes involved in the production of inflammatory compounds. As NF-kB activation has been implicated in all the stages of carcinogenesis, this transcription factor is a potential target in cancer chemoprevention and is the subject of intensive research.
The Curcumin Revolution: Greatly Enhanced Bioavailability
 Despite its impressive array of benefits, the effectiveness of oral supplementation with curcumin has been limited by poor absorption into the bloodstream through the digestive tract. In the past, a few formulators worked around this problem by adding a derivative of black pepper, piperine, which enhanced the absorption of ingredients such as curcumin.20 Scientists have long sought a more bioavailable form of curcumin to provide even greater pharmaceutical potencies to maximize curcumin’s efficacy.
Despite its impressive array of benefits, the effectiveness of oral supplementation with curcumin has been limited by poor absorption into the bloodstream through the digestive tract. In the past, a few formulators worked around this problem by adding a derivative of black pepper, piperine, which enhanced the absorption of ingredients such as curcumin.20 Scientists have long sought a more bioavailable form of curcumin to provide even greater pharmaceutical potencies to maximize curcumin’s efficacy.
Life Extension has reviewed numerous curcumin products that showed varying degrees of enhanced absorption. One published clinical study caught our attention. In it, a novel manufacturing technology was able to dramatically increase blood plasma curcumin to levels not previously seen through supple mentation. While only 50-60% of pure curcumin administered to animals is typically absorbed, this new technology increased the absorption of curcumin to a remarkable 96%.21 This impressive rise in bioavailability was achieved without the addition of piperine.
Impressed with these data and seeking to verify these findings, Life Extension then ran an objective comparison trial on human volunteers to determine if this novel curcumin could really deliver significantly greater concentrations of curcumin to the bloodstream and for a longer sustained time period.22 The findings from Life Extension’s independent study basically mirrored those in the first study. As a result, we identified a curcumin formula that provides better bioavailability than was ever thought possible. Known as BCM-95®, this “next generation” formulation is far more readily absorbed than other currently or previously available curcumin products. In fact, both studies delivered close to seven times more curcumin than was previously available from a standard supplement.
Life Extension’s study of this new formulation was designed to document the “pharmacokinetics,” or absorption, circulation, and metabolism of curcumin and curcuminoids in human subjects. The study compared BCM-95® with two other curcumin products: a plant-bound curcumin formulation with piperine and a purified 95% curcumin standalone extract (which is what most supplement companies sell today). Eleven volunteers were recruited. They were divided into three groups. Subjects received BCM-95®, plant-bound curcumin extract with piperine, or ordinary curcumin extract. Blood was drawn at baseline and again six times over the following eight hours. After a two-week washout period, subjects were switched (crossed over) to an alternate formulation; BCM-95® subjects were given ordinary “control” curcumin, while subjects originally given either of the two “control” curcumin formulations were given BCM-95®.
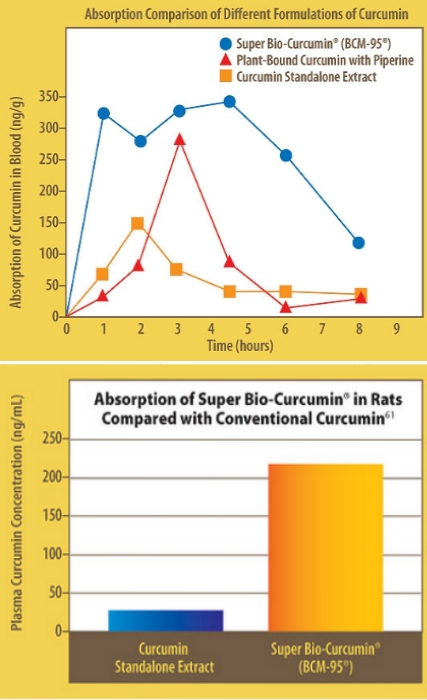
Chart 1 :Absorption comparison of different formulations of curcumin
Chart 2: Bioavailability in rats fed with BCM-95® is 7.8 times higher than conventional curcumin.
New Formulation Increases Blood Levels More, Sooner, Longer
 Subjects’ plasma samples were subsequently assayed to determine curcumin concentrations. The results clearly showed that BCM-95® was rapidly absorbed from the digestive system, allowing more of turmeric’s powerful disease-fighting chemicals to circulate throughout the bloodstream, while delivering the full punch of curcumin’s properties as never before. In fact, curcumin reached a peak within one hour in the bloodstreams of subjects who took BCM-95®. After a brief dip at about two hours’ post-dose, curcumin reached a second, still higher peak again at 4.5 hours, and then gradually declined. By eight hours’ post-dose, curcumin was still detectable in subjects’ blood.22
Subjects’ plasma samples were subsequently assayed to determine curcumin concentrations. The results clearly showed that BCM-95® was rapidly absorbed from the digestive system, allowing more of turmeric’s powerful disease-fighting chemicals to circulate throughout the bloodstream, while delivering the full punch of curcumin’s properties as never before. In fact, curcumin reached a peak within one hour in the bloodstreams of subjects who took BCM-95®. After a brief dip at about two hours’ post-dose, curcumin reached a second, still higher peak again at 4.5 hours, and then gradually declined. By eight hours’ post-dose, curcumin was still detectable in subjects’ blood.22
In contrast, ordinary standalone curcumin took two hours to reach peak concentration, and then rapidly declined. By 4.5 hours’ post-dose, when BCM-95® curcumin was just hitting its stride, curcumin from this control formulation had virtually disappeared from subjects’ bloodstreams. Even at its peak, this control curcumin formulation reached only about half the concentration of curcumin from BCM-95®. Likewise, the BCM-95® showed superior absorption compared with the plant-bound curcumin with piperine formula. Thus, BCM-95® not only delivers more curcumin to the bloodstream, sooner, but it sticks around nearly twice as long, too. This is an extremely important advantage, which should result in greatly enhanced benefits.
How did the inventors of this patent-pending curcumin achieve this breakthrough? Rather than focusing on further purification of curcumin and curcuminoids derived from turmeric (usually marketed as “95% curcumin/curcuminoids”), the formulators went back to the “roots,” so to speak, reincorporating many of the components of raw turmeric root—which are normally removed during the extraction process—and greatly enhancing the bioavailability of active constituents in the process. In essence, this revolutionary reformulation relies on the inherent synergy of the turmeric rhizome’s natural components to dramatically enhance bioavailability.
As a result, BCM-95® is six-to seven times more bioavailable than ordinary 95% extract. Just one 400 mg dose of this new bioavailability-enhanced turmeric extract is equivalent to taking 2,772 mg of standard “95%” curcumin extract or 2,548 mg of plant-bound curcumin extract with piperine. In the Life Extension human trial, BCM-95® delivered 6.93 times more curcumin to the bloodstream than the ordinary standalone curcumin product and 6.37 times more curcumin to the bloodstream than the plant-bound curcumin extract with piperine.22
Life Extension’s results confirmed the findings shown previously for BCM-95®.
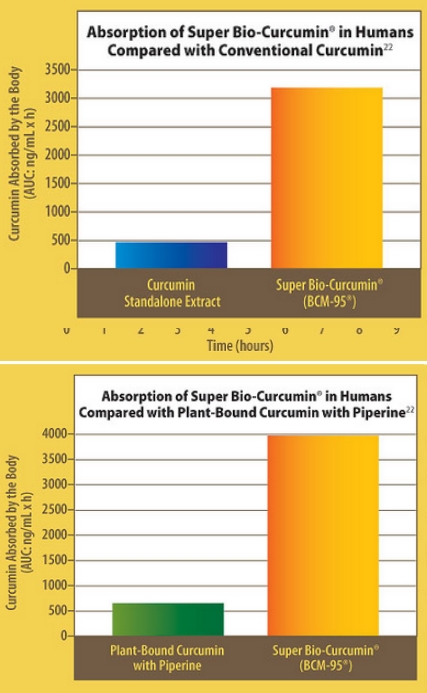
Chart 3: Super Bio-Curcumin® (BCM-95®) showed 6.9 times greater bioavailability (absorption and sustainability over 8 hours) in humans compared with conventional curcumin (as measured by the area under the curve [AUC] in a plot of blood levels against time, that is, the total amount of curcumin absorbed by the body over 8 hours).
Chart 4: Super Bio-Curcumin® (BCM-95®) showed 6.3 times greater bioavailability (absorption and sustainability over 8 hours) in humans compared to plant-bound curcumin with piperine (as measured by the area under the curve [AUC] in a plot of blood levels against time, that is, the total amount of curcumin absorbed by the body over 8 hours).
Curcumin’s Many Benefits
 Curcumin’s benefits are so diverse that they affect virtually every organ system in the body. It’s no accident that the National Institutes of Health has funded numerous studies investigating curcumin, which include diverse applications such as treatment of cystic fibrosis, the feasibility of controlling the autoimmune disease, scleroderma, and various cancer chemoprevention trials.23 Meanwhile, pharmaceutical companies around the world are actively working to derive patentable molecules based on curcumin, which they hope to market, at great profit, as anticancer treatments.24
Curcumin’s benefits are so diverse that they affect virtually every organ system in the body. It’s no accident that the National Institutes of Health has funded numerous studies investigating curcumin, which include diverse applications such as treatment of cystic fibrosis, the feasibility of controlling the autoimmune disease, scleroderma, and various cancer chemoprevention trials.23 Meanwhile, pharmaceutical companies around the world are actively working to derive patentable molecules based on curcumin, which they hope to market, at great profit, as anticancer treatments.24
Among other activities, curcumin has demonstrated antibacterial, antifungal, antiviral, anti-inflammatory and antioxidant capabilities.18 It’s even showing great promise in the fight against the most common genetic disorder in Caucasians, cystic fibrosis.14 To this list, add powerful anticancer protection, cardiovascular protection, and protection against neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases.8,16,25-29 Additionally, curcumin shows promise as a potential treatment for multiple sclerosis,7 and may protect against cataracts30 as well as reverse some of the damage associated with the high blood sugar levels that characterize diabetes.31 Curcumin also shows great promise as a treatment for skin disorders such as psoriasis, and in the treatment of wounds.9, 32
Scientists usually go out of their way to avoid hyperbole when describing the subjects of their inquiries, but curcumin’s amazing properties evidently tempt even the most staid researcher to throw caution to the wind. “Curcumin appears to possess all the desirable features of a desk-designed, multipurpose drug,” wrote one research team, recently.33 Other investigators focused on promising anticancer activity. “Curcumin… has emerged as one of the most powerful chemopreventive and anticancer agents,” wrote Indian researchers last year. “Its biological effects range from antioxidant [and] anti-inflammatory to inhibition of angiogenesis, and [it] is also shown to possess specific antitumoral activity.”27
Although anticancer drugs weaken the immune system, curcumin actually enhances it, 12,34-43 acting as an “immunorestorer.”34 It’s little wonder, then, that cancer prevention and treatment has emerged as one of the most avidly researched aspects of curcumin’s potential benefits.
Writing in Cancer Letters, American scientists noted recently, “Pre-clinical studies in a variety of cancer cell lines including breast, cervical, colon, gastric, hepatic, leukemia, oral epithelial, ovarian, pancreatic, and prostate have consistently shown that curcumin possesses anticancer activity in vitro and in pre-clinical animal models.”44 Other investigators wrote: “Carcinogenesis encompasses three closely associated stages: initiation, progression, and promotion. [Curcumin] has been shown to possess anti-inflammatory, antioxidant, and antitumor properties. [It] has also been shown to be beneficial in all three stages of carcinogenesis.”43
Specific Anticancer Mechanism Discovered
 In mid-2007, scientists at the University of Alabama at Birmingham published a report in the journal Cancer Research, detailing at least one of perhaps many mechanisms by which curcumin functions as an anticancer agent. The investigators grew prostate cancer cells in the laboratory, and exposed them to varying concentrations of curcumin. The curcumin reduced the cells’ production of a protein known as MDM2, which is associated with the formation of malignant tumors. Simultaneously, curcumin prompted the cells to produce another protein associated with the promotion of programmed cell death (apoptosis).27 Like NF-kB, an inducer of inflammation, MDM2 has been suggested as a novel target for human cancer therapy.
In mid-2007, scientists at the University of Alabama at Birmingham published a report in the journal Cancer Research, detailing at least one of perhaps many mechanisms by which curcumin functions as an anticancer agent. The investigators grew prostate cancer cells in the laboratory, and exposed them to varying concentrations of curcumin. The curcumin reduced the cells’ production of a protein known as MDM2, which is associated with the formation of malignant tumors. Simultaneously, curcumin prompted the cells to produce another protein associated with the promotion of programmed cell death (apoptosis).27 Like NF-kB, an inducer of inflammation, MDM2 has been suggested as a novel target for human cancer therapy.
To test curcumin’s effects in a live model, the scientists grafted human prostate cancer cells onto special mice, which subsequently developed tumors. The mice were then fed either curcumin or a placebo, five days a week, for four weeks. Afterwards, curcumin-fed mice were further divided into three test groups. One group continued to receive curcumin alone, while another received curcumin plus the cancer chemotherapy drug, gemcitabine. The final group received curcumin plus radiation treatment.
“Curcumin inhibited growth of [human-to-mouse prostate cancer grafts] and enhanced the antitumor effects of gemcitabine and radiation. In these tumors, curcumin reduced the expression of MDM2,” wrote the researchers. This suppression, or “down-regulation” of MDM2 expression was declared to be a newly discovered mechanism by which curcumin exerts its anticancer activity. MDM2 down-regulation by curcumin “may be essential for its chemopreventive and chemotherapeutic effects,” the scientists concluded.27
It’s interesting to note that epidemiological studies show the incidence of prostate cancer among men in India to be among the lowest in the world. One recent study estimated that the annual prostate cancer incidence rate in India ranges from 5.0 to 9.1 per 100,000/year. In contrast, among whites in the United States, the incidence rate is 110.4 per 100,000/year—more than ten times higher compared with men from India. The rate for African Americans is even higher.45 Perhaps not coincidentally, Indian men’s consistent intake of turmeric, in the form or curry, is among the highest in the world. The average intake of turmeric in the Indian population is 2-2.5 g/day, providing 60-200 mg curcumin.
Pancreatic Cancer
 Curcumin has also been shown to enhance the efficacy of the chemotherapy agent, gemcitabine, in the treatment of pancreatic cancer. Although it is currently the best treatment for this aggressive cancer, gemcitabine often loses its effectiveness as cancer cells develop resistance to the drug. Scientists from the University of Texas M.D. Anderson Cancer Center showed recently that curcumin prevents the development of this resistance, in both cultured pancreatic cancer cells and in living animal models of the disease. “Overall, our results suggest that curcumin potentiates the antitumor effects of gemcitabine in pancreatic cancer by suppressing proliferation, angiogenesis, NF-kB, and NF-kB-regulated gene products,” concluded the scientists.46
Curcumin has also been shown to enhance the efficacy of the chemotherapy agent, gemcitabine, in the treatment of pancreatic cancer. Although it is currently the best treatment for this aggressive cancer, gemcitabine often loses its effectiveness as cancer cells develop resistance to the drug. Scientists from the University of Texas M.D. Anderson Cancer Center showed recently that curcumin prevents the development of this resistance, in both cultured pancreatic cancer cells and in living animal models of the disease. “Overall, our results suggest that curcumin potentiates the antitumor effects of gemcitabine in pancreatic cancer by suppressing proliferation, angiogenesis, NF-kB, and NF-kB-regulated gene products,” concluded the scientists.46
Colon and Breast Cancers
 Curcumin’s efficacy against colon cancer has received great attention, primarily because curcumin’s bioavailability has been less of an issue, given that the colon is exposed to curcumin as it passes through the digestive tract.17 Its excellent tolerability and safety have been demonstrated in five Phase I clinical trials in colon cancer, and Phase II trials are currently enrolling patients.44 British investigators showed recently that curcumin interferes with the proliferation of various types of colon cancer, and that it enhances the efficacy of an existing chemotherapeutic agent, oxaliplatin.47
Curcumin’s efficacy against colon cancer has received great attention, primarily because curcumin’s bioavailability has been less of an issue, given that the colon is exposed to curcumin as it passes through the digestive tract.17 Its excellent tolerability and safety have been demonstrated in five Phase I clinical trials in colon cancer, and Phase II trials are currently enrolling patients.44 British investigators showed recently that curcumin interferes with the proliferation of various types of colon cancer, and that it enhances the efficacy of an existing chemotherapeutic agent, oxaliplatin.47
Curcumin’s potential role in the fight against breast cancer is nothing short of remarkable. Italian researchers reported recently that curcumin is effective against a common variety of breast cancer cells and a mutant line of cells that has developed resistance to common chemotherapy drugs. “Through analyses of the effects on cell proliferation, cycling and death, we have observed that the antitumor activity of curcumin… is at least equal in the [multi-drug-resistant breast cancer] cell line compared to the [ordinary breast cancer cell line],” wrote researchers.48 This efficacy also held true for a type of multi-drug-resistant leukemia cell.
The Italians’ research indicates that curcumin seems capable of adapting its anticancer activity according to need. “Remarkably,” wrote the scientists, “[curcumin and one of its derivatives] appeared to modify their molecular effects according to the diverse gene expression patterns existing in the [multidrug-resistant and ordinary breast cancer cell line]. Clearly, the structure and properties of curcumin can form the basis for the development of antitumor compounds…”48
Material used with permission of Life Extension. All rights reserved.
1. Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;59:51-75.
2. Araujo CC, Leon LL. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001 Jul;96(5):723-8.
3. No authors. Curcuma longa (turmeric). Monograph. Altern Med Rev. 2001 Sep;6 Suppl S62-6.
4. Limtrakul P. Curcumin as chemosensitizer. Adv Exp Med Biol. 2007;595:269-300.
5. Lin JK. Molecular targets of curcumin. Adv Exp Med Biol. 2007;595:227-43.
6. Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin. Adv Exp Med Biol. 2007;595:127-48.
7. Bright JJ. Curcumin and autoimmune disease. Adv Exp Med Biol. 2007;595:425-51.
8. Miriyala S, Panchatcharam M, Rengarajulu P. Cardioprotective effects of curcumin. Adv Exp Med Biol. 2007;595:359-77.
9. Thangapazham RL, Sharma A, Maheshwari RK. Beneficial role of curcumin in skin diseases. Adv Exp Med Biol. 2007;595:343-57.
10. Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann NY Acad Sci. 2005 Nov;1056:206-17.
11. Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105-25.
12. Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007 Jan;27(1):19-35.
13. Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006 Mar 27;78(18):2081-7.
14. Gautam SC, Gao X, Dulchavsky S. Immunomodulation by curcumin. Adv Exp Med Biol. 2007;595:321-41.
15. Funk JL, Oyarzo JN, Frye JB, et al. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006 Mar;69(3):351-5.
16. Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197-212.
17. Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471-80.
18. Osawa T. Nephroprotective and hepatoprotective effects of curcuminoids. Adv Exp Med Biol. 2007;595:407-23.
19. Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem. 2007 Feb 16;282(7):4533-44.
20. Shoba G, Joy D, Joseph T, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998 May;64(4):353-6.
21. Benny M, Antony B. Bioavailability of Biocurcumax (BCM-095™). Spice India. 2006 Sept 9;19(9):11-5.
22. Antony B, Benny M, Kaimal TNB, et al. A controlled randomized comparative human oral bioavailability of “Biocurcumax™ (BCM-95® CG)—A novel bioenhanced preparation of curcuminoids. Study submitted for publication, 2007.
23. Available at: http://rarediseases.info.nih.gov/html/reports/fy2004/niddk.html; http://rarediseases.info.nih.gov/html/reports/fy2001/orwh.html; http://rarediseases.info.nih.gov/html/reports/fy2000/nci.html. Accessed August 1, 2007.
24. Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77-103.
25. Ng TP, Chiam PC, Lee T, et al. Curry consumption and cognitive function in the elderly. Am J Epidemiol. 2006 Nov 1;164(9):898-906.
26. Lim GP, Chu T, Yang F, et al. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001 Nov 1;21(21):8370-7.
27. Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007 Mar 1;67(5):1988-96.
28. Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006 May;6(3):259-70.
29. Surh YJ, Chun KS. Cancer chemopreventive effects of curcumin. Adv Exp Med Biol. 2007;595:149-72.
30. Suryanarayana P, Krishnaswamy K, Reddy GB. Effect of curcumin on galactose-induced cataractogenesis in rats. Mol Vis. 2003 Jun 9;9:223-30.
31. Arun N, Nalini N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum Nutr. 2002;57(1):41-52.
32. Dujic J, Kippenberger S, Hoffmann S, et al. Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. J Invest Dermatol. 2007 Aug;127(8):1992-2000.
33. Salvioli S, Sikora E, Cooper EL, Franceschi C. Curcumin in Cell Death Processes: A Challenge for CAM of Age-Related Pathologies. Evid Based Complement Alternat Med. 2007 Jun;4(2):181-90.
34. Bhattacharyya S, Mandal D, Sen GS, et al. Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: protection by curcumin. Cancer Res. 2007 Jan 1;67(1):362-70.
35. Churchill M, Chadburn A, Bilinski RT, Bertagnolli MM. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. J Surg Res. 2000 Apr;89(2):169-75.
36. Pal S, Bhattacharyya S, Choudhuri T, et al. Amelioration of immune cell number depletion and potentiation of depressed detoxification system of tumor-bearing mice by curcumin. Cancer Detect Prev. 2005;29(5):470-8.
37. Perkins S, Verschoyle RD, Hill K, et al. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002 Jun;11(6):535-40.
38. South EH, Exon JH, Hendrix K. Dietary curcumin enhances antibody response in rats. Immunopharmacol Immunotoxicol. 1997 Feb;19(1):105-19.
39. Kurup VP, Barrios CS, Raju R, et al. Immune response modulation by curcumin in a latex allergy model. Clin Mol Allergy. 2007;51.
40. Xu Y, Ku B, Tie L, et al. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006 Nov 29;1122(1):56-64.
41. Kim GY, Kim KH, Lee SH, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005 Jun 15;174(12):8116-24.
42. Bhattacharyya S, Mandal D, Saha B, et al. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J Biol Chem. 2007 Jun 1;282(22):15954-64.
43. Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8(3):E443-9.
44. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007 Apr 18.
45. Hebert JR, Ghumare SS, Gupta PC. Stage at diagnosis and relative differences in breast and prostate cancer incidence in India: comparison with the United States. Asian Pac J Cancer Prev. 2006 Oct;7(4):547-55.
46. Kunnumakkara AB, Guha S, Krishnan S, et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007 Apr 15;67(8):3853-61.
47. Howells LM, Mitra A, Manson MM. Comparison of oxaliplatin- and curcumin-mediated antiproliferative effects in colorectal cell lines. Int J Cancer. 2007 Jul 1;121(1):175-83.
48. Poma P, Notarbartolo M, Labbozzetta M, et al. The antitumor activities of curcumin and of its isoxazole analogue are not affected by multiple gene expression changes in an MDR model of the MCF-7 breast cancer cell line: Analysis of the possible molecular basis. Int J Mol Med. 2007 Sep;20(3):329-35.
49. Jiang J, Wang W, Sun YJ, et al. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur J Pharmacol. 2007 Apr 30;561(1-3):54-62.
50. Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003 Jan;126(Pt 1):5-19.
51. Dairam A, Limson JL, Watkins GM, Antunes E, Daya S. Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male Wistar rats. J Agric Food Chem. 2007 Feb 7;55(3):1039-44.
52. Shukla PK, Khanna VK, Khan MY, Srimal RC. Protective effect of curcumin against lead neurotoxicity in rat. Hum Exp Toxicol. 2003 Dec;22(12):653-8.
53. Daniel S, Limson JL, Dairam A, Watkins GM, Daya S. Through metal binding, curcumin protects against lead- and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J Inorg Biochem. 2004 Feb;98(2):266-75.
54. Scapagnini G, Colombrita C, Amadio M, et al. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. 2006 Mar;8(3-4):395-403.
55. Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006 Feb;197(2):309-17.
56. Braverman ER, Chen TJ, Prihoda TJ, et al. Plasma growth hormones, P300 event-related potential and test of variables of attention (TOVA) are important neuroendocrinological predictors of early cognitive decline in a clinical setting: Evidence supported by structural equation modeling (SEM) parameter estimates. AGE. 2007; [Epub ahead of print].
57. Fiala M, Lin J, Ringman J, et al. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2005 Jun;7(3):221-32.
58. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005 Feb 18;280(7):5892-901.
59. Zhang L, Fiala M, Cashman J, et al. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2006 Sep;10(1):1-7.
60. Available at: http://www.pdrhealth.com/drug_info/nmdrugprofiles/nutsupdrugs/cur_0087.shtml. Accessed August 8, 2007.
61. Bioavailability study of BCM-95® in rats. Orcas International, Inc. 2006




